About Us
Since its start in 2000, it has remained the largest multidisciplinary and cross-institutional network of clinicians and scientists conducting neuromuscular research studies. To date over 1,300 patients have been enrolled into 19 studies.
As of 2016 the coordinating center for CINRG has transitioned to a neuromuscular specialized contract research organization (CRO), the Therapeutic Research in Neuromuscular Disorders Solutions (TRiNDS). TRiNDS is a full-service CRO with four main solutions in clinical operations, data management, biostatistics, and study measurements. TRiNDS works to monitor and establish both network and protocol specific standards to ensure the quality and safety of clinical research protocols run through the network. See www.trinds.com for more information about TRiNDS.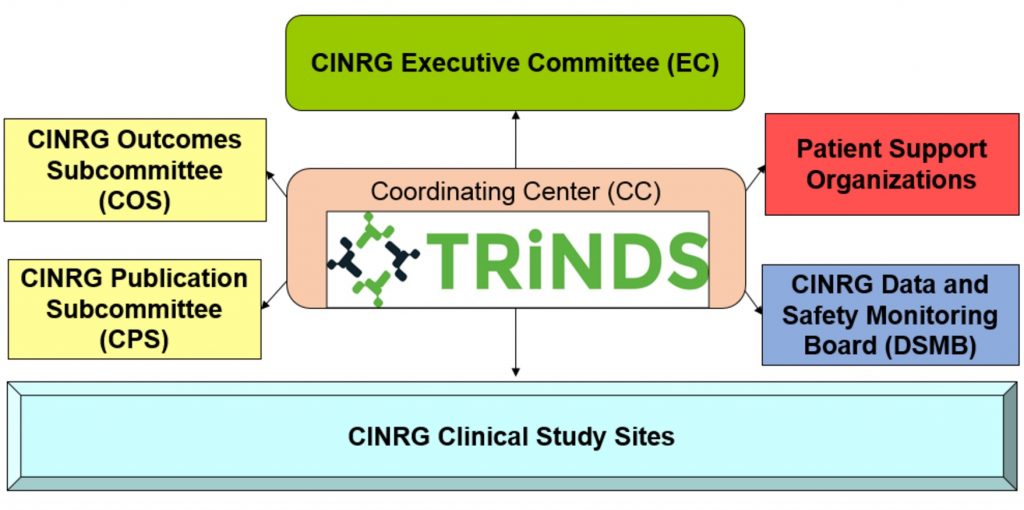
The network has a data and safety monitoring board (DSMB) which reviews all active study protocols to ensure that all studies are conducted safety and quality data is collected. The CINRG DSMB has extensive experience in clinical research and safety monitoring in a variety of disease states, including neuromuscular disorders and includes patient representatives.
